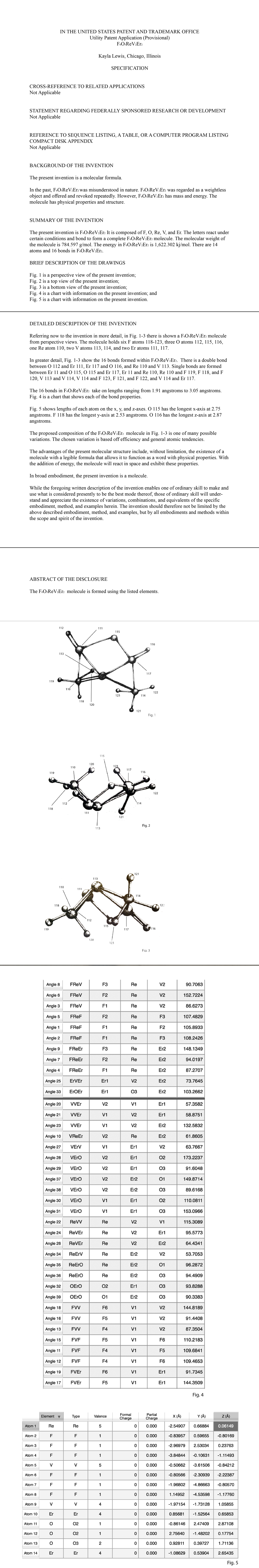
explore
F6O3ReV2Er2
F: fluorine
O: oxygen
Re: rhenium
V: vanadium
Er: erbium
O: oxygen
Re: rhenium
V: vanadium
Er: erbium
F6O3ReV2Er2
can exist in a variety of chemical arrangements. The models below distribute the atoms so that they reach the closest level of stability. Currently, the composition of
F6O3ReV2Er2
does not permit its creation in a lab: it is expensive and dangerous. Although the characteristics of the reaction in a physical environment can’t be accurately described until the word is created, we can predict the sensual experience based off chemical properties.
Under the influence of heat, the metals in F6O3ReV2Er2 would soften and one interaction would create a gray-green solid. The remaining metals would shine silvery-white and retain structure, their forms resistant to heat. Certain oxides within F6O3ReV2Er2 manipulate energy, and the reaction would take place at higher speeds.
F6O3ReV2Er2 was studied under the lens of ownership through the execution of a utility patent. The patent is currently in the drafting process and displayed below.
Under the influence of heat, the metals in F6O3ReV2Er2 would soften and one interaction would create a gray-green solid. The remaining metals would shine silvery-white and retain structure, their forms resistant to heat. Certain oxides within F6O3ReV2Er2 manipulate energy, and the reaction would take place at higher speeds.
F6O3ReV2Er2 was studied under the lens of ownership through the execution of a utility patent. The patent is currently in the drafting process and displayed below.
Wireframe molecule of F6O3ReV2Er2
Patenting F6O3ReV2Er2